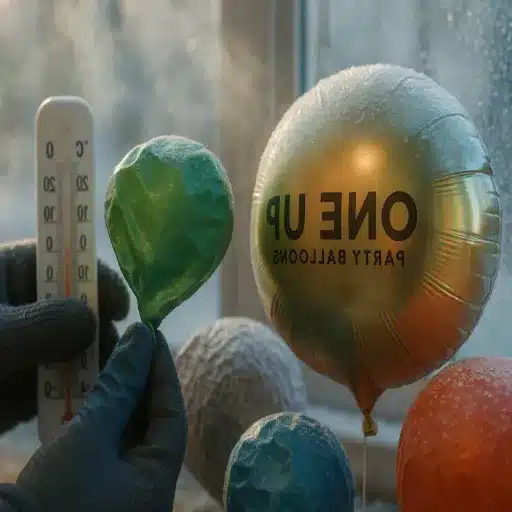Traditional helium balloons are the life and soul of every party. They bring enormous fun and a little bit of magic to any occasion. But you will have perhaps observed a rather strange aspect – helium balloons sometimes actually shrink or deflate when the air gets a little colder. Such phenomena tend to be begotten by questions. The reason for doing so is, is it merely the low temperatures acting or is there something far more involved? It is hotel information that changes of temperature and pressure may be helpful in solving simple puzzles like running out of Helium in a party as explained in the succeeding parts of this paper. I’m going to talk about the history of Helium, the effects of weather on ballooning and how to keep them flying in different situations. This research was directed towards understanding evaporation rates and understanding humidity as well as freezing or drying of the light fluid it used. If you are a mother who throws birthday parties, an interested layman or the one who simply likes balloons I promise you will have a much better understanding as to why these things that float in the air are fun after you learn a little about the subject.
The Science of Helium Balloons
Understanding Helium and Its Properties
The chemical element Helium, identified as a noble gas, happens to show absolute domination as the second lightest and second most numerous element in the world. This is due to the compound having an atomic structure that gives it near zero specific gravity within a thermodynamically stable condition free from any form of reactivity rendering it perfect for filling lighter-than-air craft such as balloons. The atomic mass of helium is in the region of 4 atomic mass units, making its density appear very low and hence it attains buoyancy objects filled with helium.
Additionally, helium is influenced by two major properties. Notably, helium is incapable of undergoing chemical reactions with other chemical species. Therefore, helium has applications that are inherently safe and cannot be burned like helium filled balloons even as an alternative to helium gas or hydrogen in filled balloons. In addition, inertness implies that the helium will easily disperse a lot faster after its release into a balloon unlike in the case of hydrogen which is largely bonding and explodes when exposed to a flame. The smaller the helium atom is, the easier it escapes, especially when dealing with materials such as latex. Yet there are materials which are more tight, like mylar, reducing the speed of diffusion and keeping the gas held within for a good time.
Being a gas, it is also important to know that helium has certain limits in its effectiveness owing to the conditions of temperature and pressure. For instance, low temperatures make helium to shrink, resulting into diminishing the volume of the balloon while high temperatures can cause contraction resulting into possible bursting of the balloon. With this knowledge, a person can have successful results working with helium-based balloons where both gravity and strength endurance are issues.
The Role of Temperature in Balloon Inflation
The activity of the inflated balloons is highly influenced by the temperature. It defines all the important characteristics like ms in terms of its size, its ability to fend against external forces as well as its overall functioning. When the balloon is exposed to cool conditions, the gas particles in the body, such as helium, lose their energy and draw together which leads to the contraction or diminished lift of the balloon. On the other hand, with the increase in temperature more energy is imparted to the gas particles and they undergo an expansion leading to an increase in material of the balloon or exertion of a larger force on the material of the balloon. This oversize leads to a constraint of the balloons structure and the possibility of it tearing, especially when a certain limit is exceeded membrane-forming bracelets such as latex because they tend also to increase in tears due to flexural deformations.
Literature says that the necessary temperature conditions must be available so that the balloon’s size remains steady as well as the elasticity of the material is also maintained. For example elastic rubbers (coesite or balloons) like helium or air are found to perform better at what can be said to be normal room temperature. This is the point at which the gas uses equilibrium due to the room temperature and very importantly the balloon bursts due to an extent of strain at the aforementioned load. There should not be huge changes in temperature hence the disadvantage of sudden shrinkage of the balloon due to cold or its bursting as a result of high pressure is to be minimised. This is crucial for people involved in activities where the accuracy of the balloons in usage such as balloon research and country field decorations is known not to interfere with the expected outcomes of those activities.
How Helium Balloons Work
When a balloon is pumped with helium, it gains general volume while exerting force against the skin of the balloon. A new lift balloon is used; remember that helium is preferred over other gases since it is classified as a noble gas and it is relatively lighter compared to any of the gases. The partial pressure of helium buoyancy works as lift because it creates a net force resultant in the helium filled balloon.
This happens because helium has lower density as compared to air resulting in a downward pressure on it at a uniform reference point adjacent to the helium balloon. Surprisingly, the gas which is the lightest of all – helium – is the most suitable for all flying objects, because it rises into the atmosphere due to a higher density gas blocking its way.
In fluid mechanics, this phenomenon is referred to as Archimedes law, which explains the relationship between weight, density, and buoyant force. The amount of this buoyant force can be found by determining the weight of fluid in which the object is submerged and hence the force the liquid can replace. When a balloon covers up all the gas it is filled with, the fluid to be displaced is air, thereby creating a difference in weight that helps the balloon rise.
Over time, helium is likely to seep out of most balloon materials because its particles are very small. Usually instead of simple latex material, a substance called Mylar™ is used which helps in reducing helium loss and increases buoyant period. Equipment, coatings and better technology are used to make on-demand helium balloons more effective and long-lasting even in specific environmental conditions.
Effects of Cold Weather on Helium Balloons
How Cold Weather Causes Balloons to Deflate
When subject to a cold environment, helium aerostats frequently possess an appearance of having deflated. This consequence, among others, is brought about by several principles underlying the nature of gases in different environments; according to the Ideal Gas Law. The lawmakers state that the volume of gas often changes in accordance with the temperature which is kept constant by a constant force. The particles of helium do not only rest down but also remove some energy from their rapid movements which make him them exert a force to the walls of balloon. In this case, the balloon walls will then depress and the balloon appears to lose air completely. It should be noted that the cold air can also alter the balloons ability to expand and can make the reduction in volume even more effective. Even though the Helium is not any more likely to escape the balloon in cold weathers as in hot climates, the balloon seems to run out of air because of the temporary shrinking. When the cold temperature regain normal one, the balloons again inflate yet again refuting the notion cold condition comes with qt most as far as gases are concerned.
Understanding the Shrinking Effect in Cold Temperatures
The reason why a cold environment induces contraction could be a consequence of the thermodynamic theory of gaseous systems, which can be particularly well explained through Charles’s Law theory. Charles’s Law states that as long as the pressure is constant, the product of temperature and volume of gas is constant. Since low temperatures affect the movement of the gas particles, the energy of gas particles that leads to the contact of the gas particles becomes more and more smaller. The climate change towards more static molecules increases temperatures- inactivity and because of that, the volume of gas in the baloon or other closed facilities will be remarkably diminished.
Different climates and levels of pressure might also increase the problematic effect that is observed. Properly sealed atmospheric pressure containers tend to remain with basic pressure compliance settings for rather moderate conditions. When sealed systems are introduced to very low temperatures, unlike the above where we were changing the volume while the pressure remains constant, the balloon will undergo subtle predetermined changes in behavior ever so slightly. Inclusive of the reduction in volume, an apparent decrease in size is further sensitized. When it comes to the reshaping of the material, attention should be given to other sources of deformation, for inholds may deform as the temperature drops and the balloon material gets cooled. Though, the most important aspect is the relationship between temperature and the resisting yield, volumetric changes in the case of a gas.
What Happens to Balloons Overnight in Cold Conditions
Because of differing material elements and structural differences between foil and latex balloons, it is an observation that each may exhibit dissimilar changes when subjected to low temperatures. The same cannot be said of latex balloons, which are usually made from synthetic latex or natural rubber. Adherence to definition of elasticity, latex is very porous and also stretches. The gas pores as well as the permeability of the rubber enhance temperature or volume changes. Low temperatures make latent rubber tubing shrink because the many gas molecules inside are losing heat – trying to make the less common in mass while the elasticity weakens the plasticity of the material.
On the contrary, foil balloons are manufactured using metallicous paper film and hence it lacks the porosity and elasticity of rubber. That is, gas does not escape as easily since it is not porous and the film tends to get wrinkled while holding its shape over a long period of time when inflated. However, it is useful to understand that temperature changes may still have an impact on some balloons in winter. That is to mean, despite metal balloons being very airtight, the gas filling may still compress due to low temperatures and they may lose their air. On the contrary, the tendency of the foil to settle amidst the reduction of internal pressure
causes some folding is in opposition to the latex balloon.
Factors That Influence Balloon Deflation
Comparing Foil Balloons and Latex Balloons
Both foil balloons and latex balloons are different as they share distinct features which affect where and how they can be used. Foil balloons are made of an incredibly thin layer of plastic and metal, making them less likely to deflate. These balloons can remain inflated for as long as a few days or even weeks especially when they contain gas. This quality makes them the best choice for decorating celebrations lasting long series of days without deflating the balloons. Besides, these balloons can be easily turned into custom branded accessories using the shiny material, making them look even more professional.
Natural rubber is the main material used to produce latex balloons. Due to its high elasticization, latex balloon enjoys an enhanced operational scope. For example, this kind of balloons is able to maintain a higher rate of deformation without losing its shape, so they are mainly used whilst creating more complex shapes and display structures. This is impeded by the fact that the latex is more permeable to air and helium gasses which causes the latex balloons to deflate more quickly than the foil balloons. Other changes to its physical properties which may be due to factors such as temperature and humidity also negatively affects the latex balloon because with low temperatures there is shrinking, which makes air leakage happen faster.
Yet the optimal choice between all these considerations is which, the event duration, the character of the need to meet some space conditions, usually compositional ideas and the requirements for the space which the balloons are supposed to occupy making sure their intended use is sustained for quite a long period of time in a same condition. Therefore, it is imperative to take into consideration this specific feature of these two types of material when choosing balloons for different occasions. Latex balloons, for example, offer more flexibility when it comes to shapes and sizes, but foil balloons are more likely to last for a longer period while reducing the need for additional charges. Both suffer poor reputation, and therefore require special handling.
The Impact of External Weather Conditions
The harshness of the weather on the outside happens to be a crucial factor if the balloons are to serve during an event and in good condition. Latex balloons, as well as foil balloons, are affected by most elements such as pressure, humidity, or wind which in turn affect the decor and normal functioning of these decorations. To take the example of balloons made of latex, extended exposure to direct sunlight may have very negative impacts leading to degeneration of balloons very fast i.e. the effect of sun rays can lead to loss of elasticity in the material through a phenomenon known as degradation due to ultraviolet radiations and making the balloon break. Besides, in the event that the heat goes up the helium gas in the balloon will also go up thereby increasing the chances of the balloon popping, especially in cases of highly nutritious balloon stuffings.
The percentage of water in the air is also relevant, which escalates the decaying process of latex through perishing due to higher than normal humidity that cause a decrease in the stretchiness of latex. On the contrary, in places with withered or low humidity, an electrostatic field can be induced where adhesion is possible thus preventing balloons from making suitable contacts or causing other incision problems. This occurs mainly in buildings with lots of walls that are properly insulated. Apart from the above-mentioned encouraging factors, there is also the prevailing factor of mechanical stress which results from the aggressive movement that might cause entanglement, abrasion or accident breakage, especially if it is an open arrangement. It is therefore necessary to compute these metrics in order to maximize the life span of the balloons.
Air Pressure and Its Effect on Balloons
The pressure of the air has a very great role on air balloons’ strength and longevity. It is known that as the air pressure of the surrounding air decreases such as on higher elevations or as found in weather systems with low pressure, the air inside the balloon will tend to increase in volume. This increase in volume often leads to excessive stretching of the balloon materials, making it more prone to tearing especially in instances when the balloon is blown close to its peak. Conversely, in the low densities reached in higher atmosphere or when there is a high atmospheric pressure present, the balloon is shrunk as the low pressure expands the balloon and it changes its proportion.
It is also important to consider the type of gas used for inflation. Helium has a lower molecular weight than the ambient air and so easily diffuses into the balloon material and peaks out faster during high differential pressure scenarios which leads to more lift loss. Moreover, modern improvements have also targeted to improve the pressure sensitivity of balloons by use of multilayer films and composite materials, thus influencing the gas pimreabily among other factors while also helping in maintaining elasticity. Mastering these dynamics is crucial for applications that range from decorative setups to scientific instrumentation, where small changes in air pressure can lead to completely different performances of the balloons.
Practical Tips for Using Helium Balloons in Cold Weather
Best Practices for Outdoor Events
In order to enhance the performance of helium balloons when used during outdoor events, especially in low temperatures, certain scientific and practical considerations will have to be met. There is a need to place expanded balloons indoors at a controlled temperature. Monitoring indoor air bubbles expands the material uniformly while also allowing for some helium volume loss that occurs when the balloon is taken out to cooler environments. No insertion is incomplete, or without some insertion of consequences. Unfortunately, the practice of storing inflated balloons for use on other occasions is not typical.
Besides that they are heavy and they don’t want to use tape to stick them to the place, and if the air pockets are obstructed, the carabiners might not be as effective. Since such times of day are known for having stronger winds, a sun balloon should be pumped up to approximately the maximum capacity of 90 percent. Such tactics do not just assure that the latex balloons filled with helium stay properly inflated and are worthwhile for a long time, but they also manage to look superb enough meeting both interesting and sensible decorative needs too.
How to Keep Balloons Inflated Longer
To enhance the buoyancy of helium filled balloons as much as possible, the use of well-chosen materials becomes imperative. Helium transmission in latex or mylar multifilms is far from being uniform. This is because unlike the case with mylar, though highly affordable, latex balloons possess some degree of microporosity and therefore do not retain helium for long periods. So for those who want to keep their party balloons afloat for as long as possible, a simple sealing treatment on the on the balloons made of latex, such as Hi-Float, will work by reducing helium permeating rate hence increase the balloon floating time by half a week. Resistance of mylar to helium gas flow is more relevant on the other hand, and this ensures that balloon float time is longer for mylar than compared to an untreated latex balloon of the same size.
In addition, environmental factors are decisive. Benefiting from sunlight and heat makes it easy for helium to expand and be lost, consequently reducing the float time. Therefore, placing the balloons in covered areas or cooler environments without extreme temperatures helps contain any such effects. Likewise, balloon steading inside buildings curtails the impact of wind and objects that may damage the balloons unlike when the balloons are freely floating. To provide more advanced options, measures were enforced which make use of different techniques such as the use of helium mix gasses to increase their longevity-although to get such results may mean doing new tests to determine the best limited pressure levels. Media performance of polymer balloons is also explained when these suggestions are used.balloon expansion operation would be less time-consuming and will allow for a more confined and suitable use of decorations for the event.
When to Bring Balloons Inside
Aside from the actual composition of the balloon, their handling process and the method wall also influences the durability of the balloons. This is due to the fact firstly helium latex or any a latex that utilises air do tend to be affected by environment settings such as double trials, or a windy weather. It is recommended to bring the balloon in once the temperatures exceed 85°F (29°C)or fall under 45°F (7°C). When the temperature is too high, helium will expand and stretch the latex, causing the balloon to burst eventually, consequently thereby (adv/con) can elast aircannots be used subject to the low the heat as the it cannot maintain the size because of the heat and it deflates. Similarly, a very high moist content although does not exhibit dryness but has latex diminishing effects in terms of durability, for instance, a measure that usually is only rectified my indoor placing.
Another thing worth mentioning is the negative effect of the sunlight on latex balloons when it is too hot. The sun’s UV rays work in a manner that shortens the durability of the balloons. In the case where the activity happens for a considerable number of hours, caution must be taken in relocating the balloons to places that receive less direct sunlight, or indoors later in the day. Controlled environmental backgrounds within a building reduce chances of such balloons deteriorating, thus making them serve for a long time as decorations.
Conclusion: Key Takeaways on Helium Balloons and Cold Weather
Summary of Key Findings
I have discovered that in popular opinion helium balloons bolsters are not very adaptable to differing environmental conditions. Concrete conditions like very low or too high temperatures noticeably change the source of the gas – helium which has the property of shrinking in such cases. VIP Balloons in Pooler, GA or appeared to be underinflated. It is a temporary outcome and those balloons will recover immediately after pressed from the side or high humidity in the room. Otherwise, the elastic limits of the material will change, and when latex is exposed to high cold there are chances of developing pores for helium diffusion and possible reinforcement loss. This is why your medium and storage should be stored in proper temperatures between 55 and 85 degrees Fahrenheit. Keeping helium balloons in such a condition means nurturing their quality and preventing them from being spoiled.
Conversely, the sun’s ultra-violet (UV) rays have the most intense destructive impact on the balloons, especially those made from latex. More specifically, ultraviolet radiation enhances polymer decomposition, a process known as photodegradation in which latex undergoes accelerated deterioration. The implication here, is that the wearability of balloons during outdoor gatherings is significantly inhibited by excessive sunlight exposure. However, it is my conviction that helium balloons ought to be brought into the indoor room or under a canopy when not in use. This will help prevent the balloons from losing their shine and integrity especially due to such factors as direct sunlight and high temperatures while at the same time enhancing the overall decorative aura of the events the balloons are used in.
Final Thoughts on Using Balloons in Different Weather Conditions
I have found evidence demonstrating that meteorological factors, to a large extent, have an impact on the quality of balloons, especially the stability of helium-filled gas balloons. For instance, such gases show changes in lengths of polymer chains of molecular films when they are exposed to ultraviolet light. When the latex film transforms into a discoloration or subsequent breakdown, the phenomenon that occurs during these changes is photodegradation. On the same note, temperature, regardless of whether it is hot or cold, can change the helium pressure in bubbles. Balloons as well as hot weather result in the increase in the size of the balloon so that it may turn into the bursting of the skin while the contraction of the gas in low temperatures would cause the air balloon to deflate ahead of its projection.
To tackle these mishaps, I propose the sound planning and preparation of the activities depending on the anticipated weather. With respect to outdoor activities, ventilate balloons only when the occasion is about to start in order to protect them from unnecessary exposure to the sun. Moreover, resistance to UV radiation or a higher gauge of latex can greatly improve the balloon’s reliability even in the least favorable conditions. Also, in the event of the spikes or drops in the temperature, crediting to the concept of another gas such as air that does not shrink and pig out as much as helium does is rather appropriate in place of helium.
The overall mixture of buoyant colors comes out well, unless rain beats that target. Always put balloons away in cool conditions, store them before an event, and use balloon weights or clips to counterbalance strong winds. Through this advanced preventive measure, the artistic and functional qualities of the placed balloons are preserved and they look attractive and also work well irrespective of nature of the weather.
Encouragement to Experiment with Balloons
Unquestionably, when you think of balloons, the list of things that can inspire you in making a more recent design with them is achieved easier. Why don’t you try the new techniques that can expand any given set of balloons not only in terms of the material they are made from but the regulation of their sizes and designs? The addition of 21st-century technologies such as eco-friendly latex and foil balloons with interesting patterns and sizes undoubtedly extends the borders of the design possibilities. For example, latex balloons are used to make large number of exhibitions through their spread around a room while foil balloons can last for a longer time and hold a tendency for themes. And everyone can see that whereas machines such as electric balloon pumps or arch kits are only few of the numerous other types of devices that can assist in creating professional designs.
Furthermore, while working with balloons, new ways can be tried and tested in enhancing the creative mechanism, such as double-stuffing to obtain unique color mixes and working with LED lights for different pieces of illumination. Among the things that can be done, for instance, one can make some organic balloon garlands, do some sculpting or put some beautiful flowers or confetti inside a clear balloon to also give some volume and look. The best part of it, you don’t need these artists to physically show you how they came up with their work. There are many video and image tutorials which can train you quite polished techniques even if you are a novice. Integrate new and old ideas, and know that the result will still remain stand-out. The new possibilities in design brought about through the initiative of experimentation, are next to appreciating on first-hand basis the craftsmanship and the technicality required in the decoration of balloons. Whether it is personal, or done for work, breaking the norms, will change the audience, as well as help in realizing the potential applications that come with balloons.
Reference Sources
- How are volume and temperature related? – Colorado State University.
- Hot and Cold Gas in Balloons – Harvard University Science Demonstrations.
- Balloons – University of Virginia.
- Lighter Than Air: Why Do Balloons Float? – University of Chicago.
Frequently Asked Questions (FAQs)
Do balloons deflate in cold weather?
Yes, balloons typically lose height in lower temperatures. This is because helium gas reduces in volume as the temperature decreases. This in turn reduces the lift of the balloon from the density of the air changes in the station surrounding the bowling. Reducing the temperature of the containing gas reduces the speed of the gas molecules thus reduction in volume and making a helium filled balloon drop close to the earth or even appears flat. This effect is more pronounced when a balloon is used in the winter or other even colder environments as compared to the room temperature. The rate at which balloons lose gas or decrease in size is also affected by the materials used to make the balloon as well as by the shape of the balloon, whether round or foil. In the event you transfer the balloon to a warmer medium, the helium gas warms up and causes the balloon to maintain its shape, often expanding and appearing normal the following day during heating.
What is the duration of helium-induced ascents per night?
The duration of the balloon’s rising ability, during the night, will also depend on the type of balloon, as well as the initial inflation and the temperature difference of the night and the morning. They lose air quite fast because the material of latex balloons is very porous and can therefore deflate significantly by the next day. Foil balloons on the other hand can keep the float time CHAVA give btye more t and CHAVA. If the balloon is taken to a colder environment, the helium will contract causing the balloon to have less lift than before. As such, in order to keep them afloat one would have to have them inside at normal room temperature. The escape of helium from helium gas filled balloons is a natural phenomenon and it is a result of the gas molecules in the balloon seeking any imperfections or small openings in the material that it has filled, so that in most cases balloons will lose a certain percentage of their content after a few days. However, this can be improved by adding some form of polymer, like Hi-Float, to the latex to provide a barrier and reduce the threat of helium loss.
Is it normal for the foiled balloons to increase in size?
Foil balloons can appear un-wrinkled for a longer period of time in comparison to latex balloons due to the metallic component in them being stiffer, however, even in this case, it is necessary to take into account – the helium obeys the laws of ideal gas and will shrink as the temperature decreases. During a temperature decrease, with the slowing down of reinforced molecular motion, the concentration of helium within the balloon also tends to reduce – this leads to the fall or wrinkle of the surface of the balloon. On a temperature increase cycle, whether normal or relatively normal, since helium does not possess weight, it has the ability to elongate the balloon to the extent that it swells back to its original shape or worst-case scenario explodes because of excessive heat. The possible changes in the shape of the balloon, i.e., the degree of inflation, after being filled with helium is dependent on the environment’s pressure and the physical structure of the balloon surface as well; plastic with precut edges differs in behavior from ordinary condoms. Balloon prepared with foil should not be used outside during cold weather since, when kept away from harsh temperatures, the volume of gas inside remains stable.
Is helium in balloon molecules affected by weather?
Any change in weather puts helium molecules to direct influence by impacting on changes that may arise in the temperature and pressure of the ambient which may impact on the buoyancy and the floating time of the balloons. In cold weather, the kinetic energy of the helium molecules is shorter such that, they occupy a smaller volume leading to the balloons appearing empty or descending lower to the ground; however, in that same cold weather, if the helium is instead raised, the gas expands, leading to an increase in pressure. This can also happen with changes in the atmospheric pressure or humidity, as these criteria also relax the balloons and enable them to provide more lift if needed. However, in most temperatures, temperature is responsible, as can be seen in the Hooke’s Law of physics. These issues are governed by the ideal gas law which explains how a ballon may hapen to shrin in a cold place and then become big again when taken to a warm place. It is guaranteed that balloons filled with helium will not become empty if they are kept at the same temperature in use and not left outside in the open for long periods in abrupt conditions.
Can the balloons still have air if one decides to keep them overnight in a car?
Letting balloons stay inside a car for an entire night can affect the volume of air within since cars go through changes in temperature which affect the helium gas and its effect on the material. Under very cold conditions, helium shrinks causing the balloons to fall down and should this cold be abruptly displaced by heat the following day, helium expands and can break the balloon or cause its seams to split. Repeated phases whereby the nights become cooler and the days warmer causes stretching of the material as a result of this that makes the balloons deflate much sooner, severely limiting the time a balloon floats. Vinyl or latex balloons are the items that are prone to this condition the most while foil balloons can maintain tension of the gas within, but there is still a problem with the pressure. To fight the loss, it is best to restrict the transportation of balloons to vehicles during the day and keep them in the usual or even higher safe warm temperatures.